It’s not even the end of the first quarter of 2022, yet inspectors with the U.S. Department of Agriculture (USDA) have documented multiple incidents of animal suffering at the hands of researchers — like a dog who suffocated to death following a surgery, five baby rhesus monkeys who died or were euthanized due to dehydration, a nonhuman primate who died during a botched anesthesia procedure, structurally unsound enclosures, and facility representatives not being available for inspections, according to USDA reports.
The following roundup is the first of what we hope will become a regular watchdog feature highlighting the horrors of animals suffering in U.S-based research facilities that have been cited for noncompliance with the federal Animal Welfare Act (AWA) by federal inspectors with the U.S. Department of Agriculture (USDA).
From Jan. 1 through March 20 of this year, seven such research facilities received “Official Warnings” from the USDA. An “Official Warning” means that the facilities are subject to civil penalties, criminal prosecution, or other sanctions for any future violations.
Lady Freethinker reached out to each company, and their responses to the official warnings, if received, are noted.
Even though modern-day science has yielded superior – and safer – human biology-based alternatives, animal testing continues due to an outdated mandate established by the federal government in 1938. It’s time for that law to change.
We hope that this series will help open our readers’ eyes to the brutal reality — and repercussions — of animal testing. Please let us know if you’d like to see more content like this by emailing [email protected].
MAYO CLINIC (Rochester, Minn.)
The Mayo Clinic-Rochester received an official warning from the USDA filed 1/27/2022 alleging a lack of adequate veterinary care stemming from a Sept. 24, 2021 site inspection.
That inspection report noted that a 9-month-old canine was found dead at the S.C. Johnson Research Facility on June 23, 2020, two days after a surgical procedure to place a tracheostomy tube into his neck. A tracheostomy involves creating a hole in the front of the neck to the windpipe, with an inserted tube keeping the hole open for breathing.
A research member had noted mucus beneath the tube on June 21 but did not report the problem to the attending veterinarian. The dog suffocated to death over the next days as a result of the large mucus plug, according to the necropsy as cited in the inspection report.
The facility has been cited with noncompliant issues at two of its 25 inspections, or 8 percent, since 2014, according to USDA records. The other cited noncompliance involved an anesthetized rhesus macaque who suffered from thermal burns on the legs after overheated fluid bags were used during an imaging procedure. The macaque fully recovered but demonstrated “evidence of behavioral stress during healing,” according to the report.
THE UNIVERSITY OF LOUISIANA- LAFAYETTE (Lafayette, La.)
The University of Louisiana at Lafayette received an official warning from the USDA filed 1/14/2022 for alleged violations of adequate housing facilities, with a note in the enforcement action that the facility must have reliable electric power and running water for nonhuman primates to drink following an Aug. 26, 2021 inspection.
The report from that inspection notes that an unresponsive infant rhesus macaque who showed signs of dehydration on July 19, 2021, was killed. Staff conducted observations “with no indication of dehydration in any of the animals in the building,” according to the report.
But the very next day, on July 20, two infant rhesus macaques also were found dead, two additional infants were unresponsive, and a third was also reported as dehydrated. A veterinarian treated the three living macaques, but only one recovered. The other two were euthanized – adding up to a total of five dead infant monkeys over the course of two days.
The report noted that problems with water pressure and the facility’s pressure regulator had caused the issues and that staff had “issued several corrective actions, including daily manual checks and records of water pressure, monthly water flow confirmation checks, water flow monitoring plan and facility water system evaluations,” according to the report.
The facility has been cited for noncompliance at three of 12 inspections since 2014, or 25 percent, adding up to four “non-critical” violations and one critical violation, according to the reports.
Other noncompliances included a female pig tailed macaque whose hind leg was fractured after a technician grabbed her for a routine physical exam, and a male pig-tailed macaque found electrocuted to death in an outdoor housing enclosure after a loose ground wire electrified the metal enclosure, according to reports.
The remaining noncompliances included more than eight macaques who escaped from their enclosures due to broken weld bars and a cracked transfer door in 2015, and also no written assurance that experiments were not unnecessarily duplicating previous experiments in 2014.
As of a May 2021 inspection report, the facility had more than 8,800 animals, including white-headed capuchins, African green monkeys, crab-eating macaques, pig-tailed macaques, rhesus macaques, and chimpanzees.
A university spokesperson told Lady Freethinker via email that after the Aug. 26, 2021 inspection, “the USDA conducted a thorough investigation and concluded that all mitigating measures implemented by the University of Louisiana at Lafayette were satisfactory.”
“The USDA concluded with only a warning, electing not to pursue any penalties or other sanctions related to this incident,” the University said. “UL Lafayette and its staff members are diligent in the care provided to animals at the New Iberia Research Center. The center follows rules and guidelines established by the U.S. Department of Health and Human Services and other agencies.”
“The report states that on July 20, 2021, a water pressure regulator malfunctioned at the New Iberia Research Center, resulting in an intermittent reduction in water pressure to an animal room housing non-human primates, circumventing the daily cage watering checks,” the University continued. “NIRC promptly reported this incident to the USDA. The NIRC has enhanced its automatic continuous pressure monitoring with an alarm to alert staff to pressure values outside acceptable ranges that interfaces with the NIRC Building Automation System. The center has also implemented additional daily manual checks and records of water pressure.”
LEGACY HEALTH (Portland, Ore.)
Legacy Health- Portland received an official warning from the USDA filed 1/14/2022 citing an alleged lack of adequate veterinary care specific to providing guidance to staff on proper handling of animals, stemming from a Sept. 23, 2021 inspection.
The inspection report noted that a nonhuman primate was being anesthetized when staff discovered that a valve on the machine had not been opened. By the time they realized the mistake, the monkey had fatal barotrauma – a term that means damage from increased air pressure – to the lungs and died.
Staff were retrained as a result of the death, according to the inspection report, which also noted that 50 animals were present at the facility, including other rhesus macaques, pigs, and sheep.
The facility has not otherwise been cited for noncompliance since 2014, according to inspection reports.
Legacy Health told LFT via email that “Legacy took appropriate action following an isolated incident that resulted in the accidental death of a primate under anesthesia.”
“The National Institute of Health’s Office of Laboratory Animal Welfare also confirmed that corrective actions taken were complete and appropriate,” the spokesperson said. “The U.S. Department of Agriculture conducted a re-inspection on Sept. 30 and found no non-compliant items. The U.S. Department of Agriculture has requested no further action.”
LAMPIRE BIOLOGICAL LABORATORIES (Pipersville, Pa.)
Lampire Biological Laboratories received an official warning from the USDA filed 2/25/2022 for enclosures that allegedly were not structurally sound following a site inspection on Dec. 14, 2021.
An inspection report from that date notes that seven donkeys escaped their enclosure in the middle of the night, and that a male donkey walked to the road, was struck by a vehicle, and died.
The facility has been cited for noncompliance at three of its 19 inspections, or about 16 percent, since 2014, adding up to one “non-critical” and two critical noncompliances, according to USDA records.
Other noncompliances included the death of a goat who was mistakenly used in a procedure where a technician removed 900 ml of blood (or nearly a quarter of a gallon) before realizing the goat was the wrong animal. The inspection report noted that the goat had not been given an exam or “been given the opportunity to go through the facility’s typical acclimation phase in which smaller volumes of blood are drawn over time.” In the hours that followed, the goat had “profuse diarrhea,” remained “in distress” even with treatment, and so was euthanized.
The USDA report noted that “the facility took immediate corrective action” and retrained all large animal technicians.
The lab also was cited in 2016 when six rabbits were directed to receive three times the maximum volume of a substance per site that had been approved in the lab’s protocols, according to the inspection report.
As of December 2021, the laboratories had more than 2,100 animals on-site, including goats, sheep, rabbits, llama, alpaca, and burros, according to a USDA inspection report.
Lampire’s President Gregory Krug told LFT via email that the agency “works tirelessly to ensure the animals under our care are treated with kindness and respect.”
“In our company’s 45 years of existence, Lampire has only had two noteworthy animal care events,” he said. “Both of these incidents were highly unusual. Both were self-reported to the USDA by our organization. Also, in both of these cases, we strongly disagree with the USDA claims and have responded accordingly.”
Lampire sent the USDA a response letter to the most recent official warning.
BLUE RIDGE KENNEL (Wetumpka, Ala.)
Blue Ridge Kennel, whose profile within the pet food industry says the company conducts feeding and palatability trials, received an official warning from the USDA filed 2/25/2022 regarding an alleged violation of adequate veterinary care following an Aug. 31, 2021 site inspection.
The inspection report from that date notes 10 issues of noncompliance, including numerous dogs with untreated medical conditions: including Isa, a female dog with a bite wound that was reddened and “appeared raw and infected,” whose condition was noted by staff but not treated until more than a week later when an inspector arrived; Cotton, an 11 year old yellow lab with an open wound on the side of his face and an ear covered with “dark colored debris” who was shaking his head during the inspection; Daisey, a 13 year old female pointer with a broken tooth, excessive tarter, and reddened gums that had gone unnoticed and untreated; and Kiss, a 12 year old chocolate lab with masses on her face, leg, and abdomen that had been observed but not treated, according to the report.
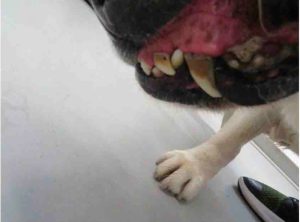
Candy. (Courtesy USDA)
The inspector also noted six dogs with moderate dental issues and one with severe dental issues, issues with fencing and excessive flies, and that a veterinarian who reportedly planned to check on several dogs with weight issues had not visited those animals nearly four months after the issue was noted, according to the inspector, who attempted to visit the vet using the address on the facility’s paperwork and found an “old site,” which redirected the inspector to a new facility not listed on the facility’s documents.
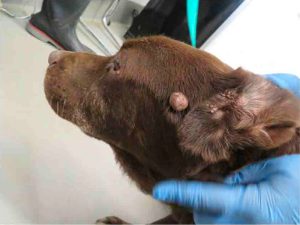
Kiss (Courtesy USDA)
The inspector concluded, “It is evident that there aren’t enough employees to carry out the responsibilities of the facility and committee.”
The company has had noncompliances at six of ten inspections, or 60 percent, since 2014 that have added up to 26 documented issues of noncompliance, including 24 deemed “non-critical” by USDA inspectors and two considered “critical,”, according to reports.
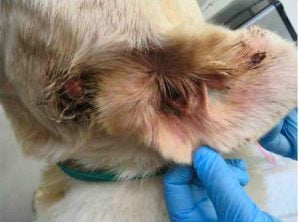
Cotton (Courtesy USDA)
The noncompliances included an Industrialized Animal Care Use Committee (IACUC) whose members “lack knowledge about the Animal Welfare Act” and were “unaware of the duties that they were required to conduct for the research facility” to ensure animal welfare, according to an April 2021 inspection report. The report also noted missing cage cards, outdated paperwork, several deep holes in the dogs’ yard that could result in injury, and cracked and peeling platforms. Also noted was chipping paint on the indoor run areas and dog beds with tears that had been “damaged beyond repair and have been for some time,” according to the inspection report. Dogs also were eating from bowls that had come in contact with rusted drying racks, according to the report.
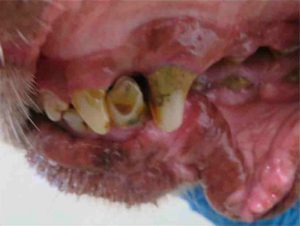
Daisey. (Courtesy USDA)
Citations in other years included that the facility records showed the facility was acquiring dogs from unlicensed individuals without verification to “ensure that the dogs are being obtained from a legal source and that random source dogs are not being used for research purposes,” according to the September 2020 report. That same report cited the facility for transporting acquired dogs without the needed health certificates, which the inspector noted “endangers the health of the animal and poses a public health risk by not ensuring the animal is free of any infectious disease,” according to the report.

(USDA inspection Report)
Other citations from 2019 noted that the IACUC hadn’t met in over a year to review the kennel’s program of animal care. The facility did not have a representative available to accompany a USDA inspector in 2017, and the facility also got cited for holes near their perimeter fencing and missing paperwork in 2015.
According to the most recently available inspection report, the facility had 96 dogs on site.
Blue Ridge Kennel could not be reached via the only contact information available online for the site.

Isa. (Courtesy USDA)
COLORADO PARKS AND WILDLIFE (Fort Collins, Colo.)
The Colorado Parks and Wildlife (CPW) received an official warning from the USDA filed 2/17/2022 following a Dec. 7, 2021 site inspection that alleged a lack of structural strength.
The inspection report notes the facility has a history of predation on prairie dogs, including by birds and bobcats, and that the one acre enclosure in which 40 prairie dogs are kept is surrounded only by a 2.5 foot high fence of chicken wire.
The facility had 78 animals on site, including moose, pronghorn, elk, black-tailed prairie dogs, bobcats, mule deer, and bighorn sheep, according to its most recent inspection report.
Colorado Parks and Wildlife Public Information Officer Travis Duncan said the citation “came as a surprise since the facility has been inspected annually by USDA without comment and facility fencing meets current USDA guidelines.”
“CPW is working with the USDA to address their concerns,” Duncan said. “The facility was modified after the December inspection and a second USDA inspection on March 2 found no non-compliances.”
TIER ONE QUALITY SOLUTIONS (Virginia Beach, Va.)
Tier One Quality Solutions, whose business profile indicates the company creates products for first responders including trauma and first aid kits and rescue bags, received an official warning from the USDA filed 3/3/2022 for allegedly not allowing access to their facility on four separate occasions.
Inspection reports from March 25, 2020; Sept. 13 and Oct. 7, 2021; and Jan 25, 2022 indicate that a facility representative was not available to receive a USDA inspector. While the USDA mandates that each research facility must be inspected at least once each year, the facility’s last successful inspection by a federal inspector was Sept. 25, 2019, according to USDA inspection reports.
The company has had noncompliances noted at five of ten inspections, or 50 percent, since 2014 on its active research license. The remaining noncompliance happened during a 2015 inspection, in which an inspector noted the company had moved their location to a new address without notifying the USDA and that inspectors could not review the records they needed regarding the animal care program.
TAKE ACTION
If you haven’t already, sign Lady Freethinker’s petition supporting the FDA Modernization Act, which would end FDA requirements established in 1938 to test on animals by validating safer, human-biology-based methods.
SIGN: Stop Requiring Cruel, Unreliable Animal Tests for US Pharmaceuticals
Also sign our petition demanding that Congress nix a proposed $30 million increase to the National Institutes of Health to breed more monkeys for lab use.
We’re also petitioning the European Commission to sign off on a demand from the European Parliament to ban animal testing as soon as possible.
SIGN: End Cruel, Needless Animal Testing in the European Union